



Next: Requirements
Up: Photo Resist Stripping
Previous: Photo Resist Stripping
Contents
Index
For our discussion we will treat the photoresist usually as a
polymer consisting of carbon (C) and hydrogen (H), the other
species present in resist are of so low concentration that
they can be ignored (maybe not always for contamination issues,
but for the topic here). In plasma assisted resist removal
there are to basic reaction ways to form volatile reaction
products:
- Oxidation:
- here the resist polymer is oxidized, the basic
reaction is:
- Reduction:
- here the resist polymer is reduced by
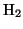 , the basic reaction is:
, the basic reaction is:
more commonly used is 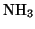 :
:
Today in nearly all ashing processes oxygen in one or the other form
(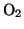 , Ozone
, Ozone 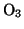 ,
, 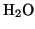 ) is used to oxidize the resist
and transfere it to gasenous species which are pumped away. Reducing
chemistries were proposed time by time, but they are not widespread
in mass production because of safety concerns. Technically they would
be interesting because some materials redeposited from the etching
process like Ti form nonvolatile oxides that could be avoided by
reducing chemistries. The rest of the section will deal,
due to the importance of the oxidizing processes, primarily with those.
) is used to oxidize the resist
and transfere it to gasenous species which are pumped away. Reducing
chemistries were proposed time by time, but they are not widespread
in mass production because of safety concerns. Technically they would
be interesting because some materials redeposited from the etching
process like Ti form nonvolatile oxides that could be avoided by
reducing chemistries. The rest of the section will deal,
due to the importance of the oxidizing processes, primarily with those.




Next: Requirements
Up: Photo Resist Stripping
Previous: Photo Resist Stripping
Contents
Index
back to www.gs68.de
Other important things: Legal Notice, Contact us
Copyright © 2001-2012 Spitzlsperger Technologies GmbH