



Next: Collision Parameters
Up: Gases and Ideal Gases
Previous: Kinetic Gas Theory
Contents
Index
Maxwell-Boltzmann Distribution
The kinetic energy distribution of electrons, ions and
neutrals is very important for characterizing a gas.
From statistical arguments and also from experiments
the so called Maxwell Boltzmann distribution can be
derived (see fig. ![[*]](crossref.png) ). This indicates
the portion of the gas constituents having a specific
velocity
). This indicates
the portion of the gas constituents having a specific
velocity 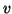 in thermal equilibrium (
in thermal equilibrium (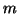 denotes the mass
of the particles). At
www.gs68.de
you can plot a Maxwell Boltzmann Distribution online.
denotes the mass
of the particles). At
www.gs68.de
you can plot a Maxwell Boltzmann Distribution online.
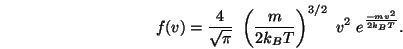 |
(1.7) |
Figure:
Maxwell Boltzmann distribution
|
|
The shape of the distribution gets wider for higher
temperatures and also the peak decreases. It is possible
to calculate a mean speed 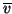 which is equal to:
which is equal to:
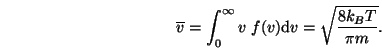 |
(1.8) |
The mean speed is always higher than the most probable
speed due to the skewness of the distribution.
For Argon atoms at 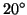 C (=295K)
C (=295K)  = 394m/s.
= 394m/s.




Next: Collision Parameters
Up: Gases and Ideal Gases
Previous: Kinetic Gas Theory
Contents
Index
back to www.gs68.de
Other important things: Legal Notice, Contact us
Copyright © 2001-2012 Spitzlsperger Technologies GmbH
![[*]](crossref.png) ). This indicates
the portion of the gas constituents having a specific
velocity
). This indicates
the portion of the gas constituents having a specific
velocity ![]() which is equal to:
which is equal to:
![]() C (=295K)
C (=295K) ![]() = 394m/s.
= 394m/s.